Page 56 - Micro5 Brochure 2017
P. 56
Effect of microcurrent electrical stimulation on tendon healing 111
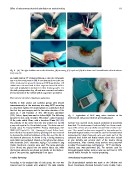
Fig. 1 (A) The right Achilles tendon after dissection, (B) tenotomy, (C) repair and (D) skin closure and immobilization with window at
tenotomy site.
and ankle held in 45° of plantar flexion so that the calf muscle
was in a shortened position [20]. A window was done at the site
of the tenotomy for wound dressing and MES application. All
rabbits were returned back to their cages and were fed ad libi-
tum with prophylactic antibiotic to their drinking water. On
the sixth postoperative day, all cast were removed and unlim-
ited movements of the rabbits within cages were permitted.
Microcurrent electrical stimulation application
Rabbits in both anodal and cathodal groups were treated Fig. 2 Application of MES using active electrode at the
transcutaneously at the tenotomy site using MES according tenotomy site and ground electrode proximally placed.
to a treatment regimen of 6 sessions/week on a daily basis from
the first day post surgery and for the entire duration of the tendons were exposed under general anesthesia as previously
study (3, 5 and 8 weeks). A Trio 300 electric stimulator described. The tendons were freed carefully from the surround-
(ITO, Tokyo, Japan) was used to deliver MES. The following ing and the sutures were carefully removed before tendon exci-
parameters were used; intensity 100 lA/cm2, pulse frequency sion. The excised tendons were assigned for biomechanical or
10 Hz, pulse width 50 ms, with a duration 30 min [8,13,14]. histopathological studies. For tendons used for biomechanical
The polarity of the active electrode was positive for anodal measurements, Sharp transverse cuts were made part of the
group and negative for the cathodal group. The device was cal- calcaneal bone below and fleshy muscles above were incised
ibrated using EZ Digital 60 MHz Analog Oscilloscope OS- to give stability and prevent slack of the tendon during
5060A (EZ Digital Co. Ltd., Gyeonggi, Korea). Before treat- measurements. After removal, those tendons were preserved
ment the skin was cleaned and any growing hair was removed in saline 9% concentration and freezed at À70 °C until biome-
to decrease the electrical resistance of the skin over the site of chanical tests were performed [21]. For tendons used for
the electrode placement. As shown in Fig. 2 during treatment, histopathological studies, sections were cut and fixed in 10%
each rabbit was positioned relaxed on his side and two dispos- neutral buffer formalin for routine processing.
able electrodes (ECG electrodes Ag/Ag Cl (Leonhard Lang
Gmbh, Innsbruck, Austria), were used. The active electrode
(1.0 · 1.0 cm) was placed over the tendon injury site, while
the inactive electrode was placed proximally on the thigh re-
gion of the same side, approximately 3 cm apart.
Tendons harvesting Biomechanical measurements
According to the assigned time of each group, the cast was The Biomechanical analysis was made at the Cellulose and
removed and the animals were weighted. The right Achilles Paper Department, National Research Center, Dokki, Cairo,